The presence of traces of N-Nitrosamine impurities in pharmaceutical products was first noted around 2018 in some sartans, particularly sartans that contained a tetrazole ring in their chemical structure and specifically Valsartan. It soon became apparent that nitrosamines were also being observed in non tetrazole containing sartans and then other pharmaceutical actives that were not even sartans e.g. Ranitidine and Metformin plus quite a few others. This was seen as a huge problem as N-nitrosamines have the potential to be carcinogenic so, as with other carcinogens or suspected carcinogens, they need to be very strictly controlled. FDA issued a guideline on this topic in September 2020 that has undergone two revisions, so the current guideline was issued in September 2024. Guidelines on this topic have also been issued by Health Canada, European Medicines Agency, and WHO. This article discusses some of the features of these regulatory guidelines and particularly highlights some of the challenges likely to be encountered.
What Are N-Nitrosamines?
N-Nitrosamine impurities are generated by nitrosation reactions between amines and nitrites e.g. sodium nitrite as shown in Figure 1.
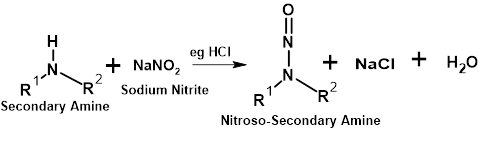
Figure 1 – Schematic Showing Nitrosation of Secondary Amine with Sodium Nitrite
Secondary amines are most susceptible to nitrosamine formation but there have been reported observations in tertiary and even quaternary amines although to be possible, this would require initial or concerted dealkylation step(s) in the reaction cascade. Primary amines are not able to form nitrosamine derivatives as reactions with nitrites lead to the formation of diazonium ions that are quite unstable and under ambient temperature conditions will fairly quickly degrade with the elimination of nitrogen.
Since N-nitrosamines are potential carcinogens, their formation in pharmaceutical products is highly undesirable and so observations of their occurrences around 2018 led to considerable concern within the industry and regulatory agencies.
Carcinogenic impurities in pharmaceutical products must be very strictly controlled. For non carcinogenic related substances type impurities, recommendations for acceptable specification limits are provided in ICH Guideline Q3A(R2) for active pharmaceutical ingredients (APIs) and Q3B(R2) for drug products. So for drug products for which maximum daily doses fall in the range of 10mg to 2g (which would cover a very wide range of products), the identification threshold for a new impurity is 0.2%. This means that for these products, the product specifications for related substances should set a limit at 0.2% for individual unknown impurities.
For impurities that are carcinogenic or suspected carcinogens, it’s quite different. These materials are extremely dangerous even in very low quantities so most regulatory guidelines are indicating that in the absence of a toxicology derived known safe level, a general no effect level (NOEL) be applied and this should be 26.5ng per day. So for a product with a unit strength of 250mg which may be administered up to 4 units daily, the maximum daily dose of 1g may not contain more than 26.5ng of carcinogenic/suspected carcinogenic impurity that does not have an established safe level. For this example, this is equivalent to 26.5ppb which is an extremely challenging level to achieve for any analytical method and this will be further discussed later.
Some Background to the N-Nitrosamine Issue
Early observations of nitrosamine impurities were in sartans (angiotensin II receptor blockers) that contain tetrazole rings in their chemical structures e.g. Valsartan (shown in Figure 2) which was found to contain traces of N-nitrosodimethylamine (NDMA)
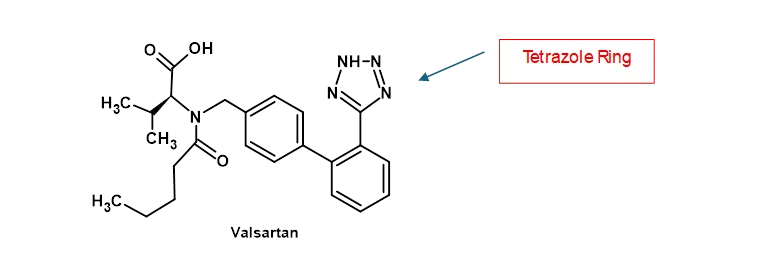
Figure 2 – Chemical Structure of Valsartan
It was initially believed that it was the chemistry around tetrazole ring synthesis that was a major cause of the problem. Sodium Azide is a commonly used reagent for tetrazole formation but it is important to remove reagent residues after the reaction is completed. Excess azide residues are generally destroyed by adding sodium nitrite to the reaction mixture and it was believed that reaction of this quenching reagent with other amine residues present in the mix led to trace amounts of nitrosamine. Although this may well have been one explanation, it rapidly became apparent that it was not the only root cause.
Due to increasing alerts that led to more laboratory investigations, trace amounts of nitrosamines were also found in:
- Sartans products that did not contain tetrazole rings
- Ranitidine
- Nizatidine
- Metformin
- Etc, etc.
These observations led to batch recalls and it became apparent that with this rapid accumulation of nitrosamine reports, we were facing a risk of creating drug shortages in affected products. So some rapid actions were needed here.
Initial observations may have suggested that nitrosamine formation was due to side reactions during synthesis and therefore they were considered to be manufacturing by-products. However, as more observations accumulated, it became clear that it was a broader issue.
As indicated previously, in order to create nitrosamines you need a secondary amine (tertiary and quaternary amines could also be sources but less efficient) and nitrite ions. These could come together in various ways i.e.
- Sources of amines during synthesis
- Starting materials
- Intermediates
- Finished API
- Synthesis by-products
- API degradation products
- Reagents
- Solvents
- Sources of Nitrites
- Synthesis reagents
- Trace impurities in pharmaceutical excipient ingredients
- Leachables in packaging
So nitrosamines could certainly be generated as manufacturing impurities. But since there was potential for the amine component to be provided in API and nitrite component to be separately provided in one or more excipient ingredients, they could also develop more slowly during the shelf life of drug products which made them a topic to be addressed during stability testing and therefore of concern to StabilityHub. In addition, it should be noted that other degradation products that are generated during product shelf life could also be susceptible to nitrosation. Also to make this way more difficult, this would not be just 1 or 2 specific molecules that need to be checked for but any molecule containing a secondary or higher order amino function could be susceptible. Since acceptable limits for nitrosamines were at ppb scale, the presence of just ppm levels of amines and nitrites could be sufficient to lead to unacceptable amounts of these carcinogens.
In order to cope with this, regulatory agencies required companies to conduct a 3-step mitigation strategy on all products which includes the following actions:
- Risk assessments on all their products which is essentially a paper exercise.
- In those cases deemed necessary, this was to be followed by confirmatory testing.
- Development, implementation and process variations could then be required.
The “3-step mitigation strategy” nomenclature is FDA terminology although the same approach is required by other agencies also.
Early regulatory guidelines on this subject tended to concentrate on a small group of specific nitrosamines as indicated below in the 7 examples that are listed in FDA August 2020 guideline:
- Nitrosodimethylamine (NDMA)
- Nitrosodiethylamine (NDEA)
- Nitrosomethylphenylamine (NMPA)
- Nitrosodiisopropylamine (NDIPA)
- Nitrosoisopropylethylamine (NIPEA)
- Nitrosodibutylamine (NDBA)
- Nitroso-N-methyl-4-aminobutyric Acid (NMBA)
The same group of nitrosamines is listed in the guidelines issued by Health Canada, EMA and WHO although these guidelines do indicate that drug substance related N-nitroso derivatives should also be considered. The June 2020 EMA guideline includes a whole section on this topic.
In September 2024, FDA issued a 2nd revision of their guideline, and this provides more information on the nature of nitrosamines that should be considered. So we now have a category that is called Small-Molecule Nitrosamines which would include the 7 examples listed above and a separate category called Nitrosamine Drug Substance-Related Impurities (NDSRIs). This is a very important revision and more details will be provided later in this article. In August 2023, FDA issued a separate guideline, Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) and this will also be further discussed later.
Just as you are getting the hang of the thought that we need an amine and some nitrite in order to get a nitrosamine problem, I can spoil that model by pointing out that you can also create a nitrosamine impurity in the complete absence of any nitrites. This has also been briefly noted within the FDA September 2024 guideline but does require unusual circumstances. For this to work we need a drug substance that contains a hydrazine group in its chemical structure. Hydrazine groups are admittedly very unusual features of pharmaceutical actives, probably because they also often lead to carcinogenic properties. However, they do happen rarely, and a good example is the Parkinson’s drug Carbidopa as shown in Figure 3.
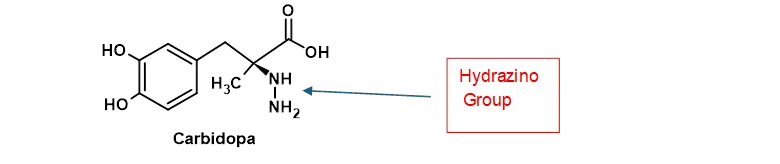
Figure 3 – Chemical Structure of Carbidopa
Simple oxidation will convert a hydrazine group to an N-nitrosamine as shown in Figure 4.
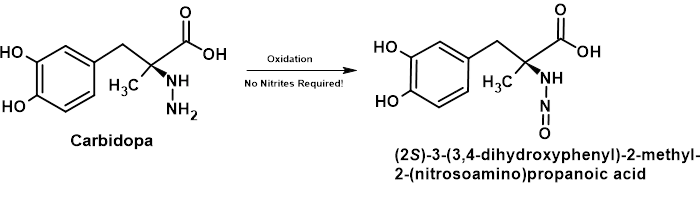
Figure 4 – Schematic Showing Nitrosation of Carbidopa by Oxidation
The Regulatory Agencies do a great job in trying to keep us all well focused on the way that we conduct our product development activities but sometimes we just have to do some of our own assessments to ensure that we are achieving the standards being set for our own specific pharmaceutical products.
More Recent Developments
Some Classification of N-Nitrosamine Impurities
FDA introduced the terminology of Small Molecule Nitrosamines and NDSRIs in their August 2023 and September 2024 guidelines
Small Molecule Nitrosamines
This category includes the 7 compounds previously listed as well as similar types of molecule that are not related to the API. The majority of examples of nitrosamines that have been observed in batches of pharmaceutical products that led to recalls are probably included in this category. The parent amines of these listed carcinogens include secondary and tertiary amines that may be used as reaction solvents and for which residues in batches would be susceptible to nitrosation by nitrites (after dealkylation for tertiary amines) perhaps during synthesis or possibly by nitrite residues in drug product excipients during shelf life. The rationale behind some cases may be less clear e.g. Nitroso-N-methyl-4-aminobutyric Acid (NMBA) may appear to be out of place here.
However, this compound has been reported in batches of product and is derived from the very commonly used synthesis solvent N-methyl pyrrolidone as follows:
- Hydrolysis of the γ-lactam to form N-methyl aminobutyric acid
- Nitrosation to NMBA
As shown in Figure 5
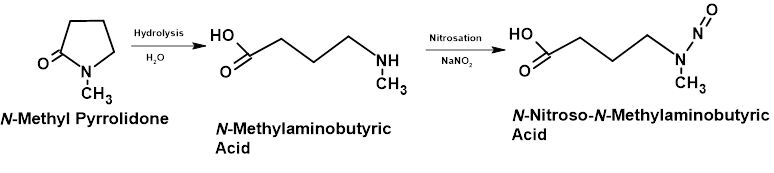
Figure 5 – Schematic Showing Hydrolysis and Nitrosation of N-Methyl Pyrrolidone
Nitrosamine Drug Substance-Related Impurities (NDSRIs)
Amino groups are structural features of huge numbers of different active pharmaceutical ingredients (APIs). Indeed many APIs contain multiple amine functions and if this were not sufficient, we also have equally huge numbers of amino groups within our API chemical structures many of which are susceptible to hydrolytic degradation to generate even more amines that are potential targets for nitrosation. In the previous section, I offered the opinion that small molecule nitrosamines probably represent the majority of observations in pharmaceutical products but in reality I think it is more likely that it is the NDSRIs that are actually present in larger numbers but they are more difficult to detect so there have been less reported observations.
In spite of this, it was a NDSRI that was one of the earlier observations of a nitrosamine impurity and it was actually seen in Valsartan which was the product that initiated this whole issue. So although small molecule nitrosamines are more frequently seen, the degradation cascade depicted in Figure 6 has been reported.
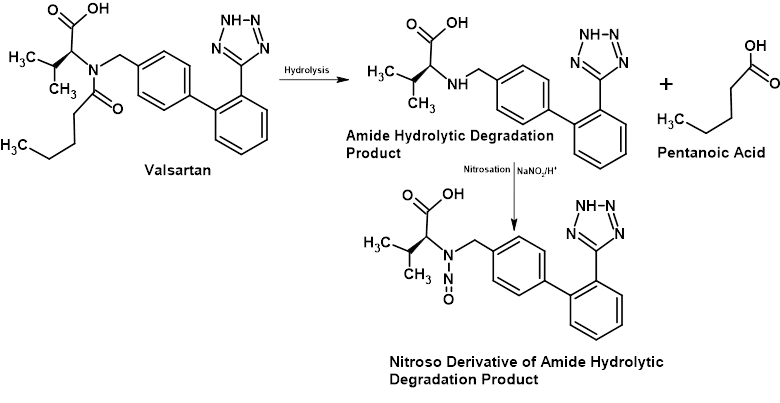
Figure 6 – Schematic Showing Hydrolytic Degradation of Valsartan Tertiary Amide Centre Followed by Nitrosation of Resulting Secondary Amine
Most regulatory guidelines that cover nitrosamine impurities do recognize that API related impurities need to be considered. However, both FDA and Health Canada have taken this a step further by providing listings of hypothetical and known NDSRIs (FDA terminology). The FDA listing is associated with their guideline, Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) that is dated August 2023 and may be found at CDER Nitrosamine Impurity Acceptable Intake Limits | FDA
This Table includes about 285 entries and can be exported into an Excel table. An extract of this Table (Excel version) is depicted in Figure 7
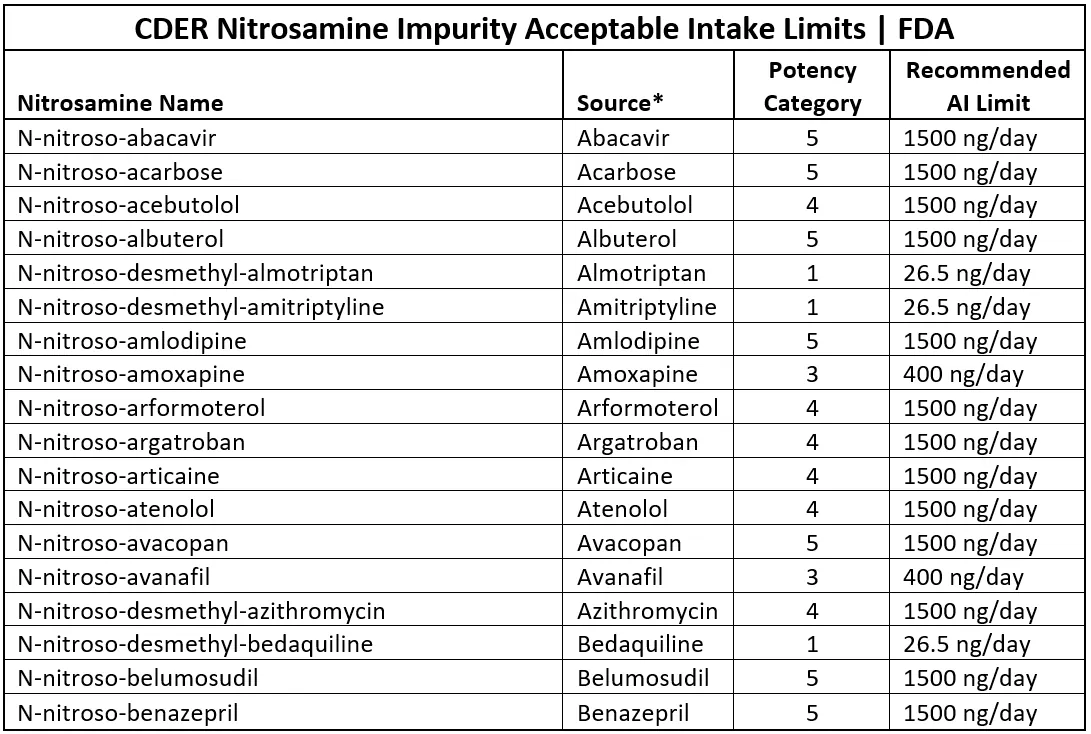
Figure 7 – Extract From Excel Version of FDA Listing of Nitrosamine Impurity Acceptable Intake Limits
Most of the listed items are straightforward nitroso derivatives of the API but some of them are derivatives of impurities e.g. item 5 which is N-nitroso-desmethyl-almotriptan in the API Almotriptan. The on-line version of this list provides access to chemical structures of the various nitrosamines by clicking on the nitrosamine name. For each nitrosamine, the table assigns a Potency Category and recommended Daily Acceptable Intake Limit which will be further discussed later.
Health Canada also provides a tabulation of nitrosamine impurities associated with their guideline, Guidance on Nitrosamine Impurities in Medications dated May 2024. This is similar to the FDA listing shown above but not exactly the same. The Health Canada version includes about 160 entries, provides chemical structures plus Carcinogenic Potency Categorization Approach (CPCA) (which is the same as FDA’s Potency Category) and Daily Acceptable Intake Limit. The link to the Health Canada listing is found at: Nitrosamine impurities in medications: Established acceptable intake limits – Canada.ca
Figure 8 provides a screenshot of an extract of this Table
Fig 8 – Screenshot of Part of Health Canada Table of N-Nitrosamine Impurities
The 2 agency tabulations do contain different entries, so it is worth checking out both of them. For example, the Valsartan NDSRI that is depicted above in Figure 6 is included in the Health Canada listing but not in the FDA table. But I believe that both agencies maintain updates of these tables so it is best to open the tables from agency websites each time they are used.
These tables should be very helpful to the industry as they provide good information particularly to generics companies but also for companies developing new molecules they do provide helpful guidance.
Establishment of Acceptable Intake Levels
The FDA and Health Canada N-nitrosamine tables provide information on the Potency Category and Recommended Acceptable Intake Levels (RAILs) for each impurity listed. Establishment of these values has been another more recent development that is described in both FDA and Health Canada 2024 guidelines.
Toxicology testing for carcinogenicity could be applied in each individual case including use of Ames testing (Bacterial Reverse Mutation Testing) as well as animal testing. The Ames Test is considered sufficiently reliable for Small Molecule Nitrosamines but because of the complexity of substituents in many NDSRIs, the reliability in this category is questionable. There is an Enhanced Ames Test available as described in the Health Canada May 2024 guideline that attempts to overcome these difficulties.
Another approach in the FDA and Health Canada tables applies Predicted Carcinogenic Potency Categorization which is based on Structural Activity Relationship (SAR) calculations. It has been determined that the carcinogenic properties of N-nitrosamine molecules are dependent on chemical structural features such as:
- Number and nature of proton distributions on C-atoms that are located α to the N-nitroso group
- Presence of activating or deactivating features within the chemical structures of the NDSRIs
Based on these 2 features, a scoring system is applied which will lead to a final result of 1 to 5. A score of 1 would indicate maximum carcinogenic activity and 5 would indicate minimum carcinogenic activity (although molecules in this category are still very strictly controlled). Recommended Acceptable Intake Levels for the 5 categories are shown in Figure 8.
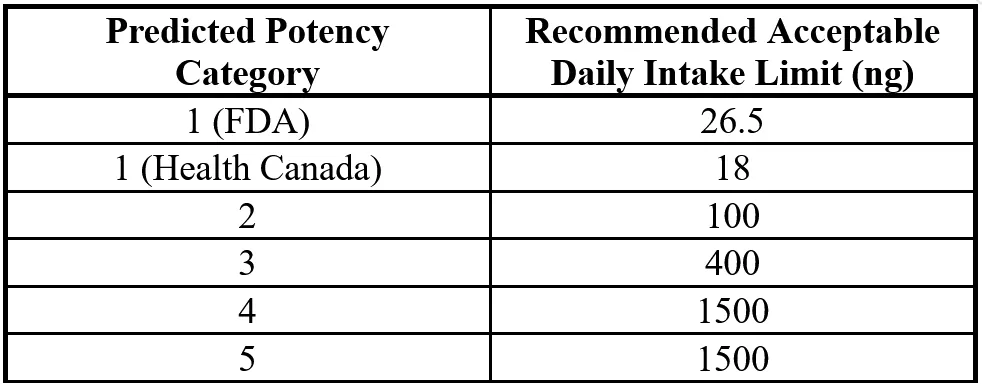
Figure 8 – N-Nitrosamine RAIL Values for Predicted Potency Categories
Note the slight difference in daily intake limits between the 2 agencies for Potency Category 1 which is 26.5ng for FDA and 18ng for Health Canada.
1500ng is the maximum value allowed and applies to both Potency Category 4 and 5. This value is assigned to be consistent with requirements of ICH Guideline M7 that specifies 1.5µg per day as Threshold of Toxicological Concern for any unstudied chemical that poses a negligible risk of carcinogenicity.
So, some further detail is required on how nitrosamine chemical structural features are applied to decide on potency category as follows:
Number and nature of proton distributions on C-atoms that are located α to the N-nitroso group (α-Hydrogen Score)
C-atoms around a nitrosamine are as indicated in Figure 9
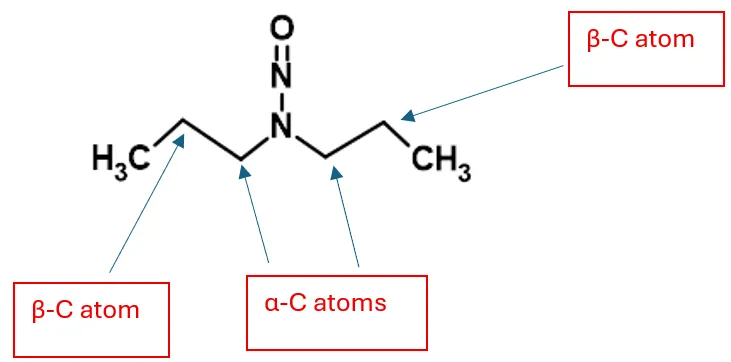
Figure 9 – Location of α- and β-C Atoms in Nitrosamine Chemical Structures
FDA and Health Canada provide some representative examples of proton distribution and Figure 10 indicates how this factor is applied
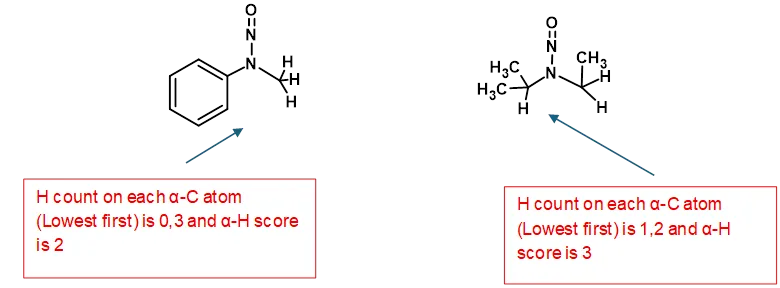
Figure 10 – Examples of Application of α-Hydrogen Score
Presence of activating or deactivating features within the chemical structures of the NDSRIs
Both FDA and Health Canada guidelines provide good details on this item. This includes the same tables of structural features that lead to deactivation or activation of carcinogenic activity. Some examples of deactivating features are:
- Presence of a carboxylic acid group
- Nitroso group in a pyrrolidone ring
- Nitroso group in a 6-membered ring that includes a sulphur atom
- Etc
The presence of these features lead to adjustments of Potency Category values of +1 to +3
Examples of activating features are:
- An aryl group attached to a α-C atom
- A methyl group attached to a β-C atom
These are the only examples provided, and in both cases lead to adjustments of Potency Category values of -1.
The overall values of α-H score + deactivation/activation score provides the Predicted Potency Category and examples are provided in FDA and Health Canada guidelines to demonstrate the procedures. There are toxicology prediction software packages commercially available that apply these SAR calculations and for more details it is recommended to check out www.lhasalimited.org.
Analytical Chemistry Challenges
So, we now have some quite effective tools available for us to make decisions on whether or not the amount of nitroso impurities present exceeds acceptable levels, but we are still left with the problem of figuring out whether our drug products do contain any nitroso impurities and if so, what are their chemical structures so that these tools can be applied. These are analytical chemistry problems and need to be addressed on freshly manufactured batches (release testing) as well as the possibility of their development during product shelf life (testing of stability samples).
As previously discussed, in accordance with ICH guideline Q3B(R2), we are generally working with specification limits at around 0.2% for new regular related impurities and this is usually quite achievable using HPLC with UV detection. When we have to deal with nitrosamine impurities with limits around 26.5ppb which is equivalent to 0.00000265%, this is likely to be way below the detection limit for most HPLC methods, so we have to use quite different technology here. Fortunately, liquid chromatography with mass spectroscopy detection (LC-MS) is now readily available and is generally quite capable of operating at these levels. The problem is that it’s OK to operate at this type of sensitivity level when you know what you are looking for, but when you are dealing with unknowns, it’s likely to get very complicated.
When dealing with unknowns, it is likely that we will need to operate the MS detector in Total Ion Current (TIC) mode. This will then record all components present which will include the target of the search (nitrosamine impurities), but also other impurities that may be present that are not nitrosamines and have a specification limit of 0.2%. So, this could generate chromatograms with very large numbers of peaks although ion mass information will be provided for each component, which may help. For example, Specific Ion Monitoring (SIM) spectra may then be collected and fragmentation patterns analyzed to establish chemical structures. One possibility is to look for fragment ion peaks that are created due to losses of -N=O (30 mass units) from the molecular ion which would indicate that the molecular ion peak was probably due to a nitrosamine. These components may then be further studied in order to establish complete chemical structures. This all represents a very substantial interpretation burden for our analytical chemists and will require significant additional training for them.
Since for any specific drug substance, potential nitrosamine impurities could include one or more direct drug substance nitrosation products depending on the number of suitable amino groups present as well as nitrosated degradation products. This adds an additional level of complication but some assistance with this may be achieved by use of Zeneth software. This is a chemistry based expert system that can predict the degradation products of organic molecules and is also available at www.lhasalimited.org
Applying specification limits based on FDA and Health Canada predicted potency category approaches still provides problems. Although category 1 requires application of the very demanding limit of 26.5ng per day of nitrosamine impurity, the limit associated with categories 4 and 5 are still very challenging. 1500ng per day is equivalent to 0.00015% which is still way below the ICH Q3B(R2) limit of 0.2% so will still require the application of LC-MS approaches and is likely to deliver very complicated chromatograms that will need to be interpreted.
Conclusions
The discovery of N-nitrosamine impurities in some pharmaceutical products in recent years has opened up areas of concern that are likely to be extremely difficult to satisfactorily address. Regulatory agencies have quite correctly attempted to deal with this issue very seriously as this class of impurities has the potential to be carcinogenic and so must be very strictly controlled.
A big part of the problem is that the term nitrosamine could cover a huge number of different impurity molecules as there are a vast number of pharmaceutical actives that contain secondary or higher order amine groups in their chemical structures as well as amide groups which have the capacity to generate amines by hydrolytic degradation. In addition, nitrite ions are also very commonly present in trace ppm quantities in many pharmaceutical excipient ingredients.
Regulatory agency guidelines and particularly those issued by FDA, Health Canada and EMA have attempted to explain the issues and propose some remedies that will present additional burdens to pharmaceutical companies but if these guidances are not followed well, we are likely to see increasing numbers of batch recalls which could impact the supply chain and create drug shortages.
References
Control of Nitrosamine Impurities in Human Drugs, Revision 2, FDA, September 2024
Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs), FDA, August 2023
Guidance on Nitrosamine Impurities in Medications, Health Canada, May 2024
Nitrosamine Impurities in Human Medicinal Products, EMA, June 2020
WHO Good Manufacturing Practices Considerations for the Prevention and Control of Nitrosamine Contamination in Pharmaceutical Products, WHO Draft Working Document, April 2024
Share This Article with the Stability Community!
February 28, 2026
Ben Was Right QC and analytical labs are facing heightened expectations as method lifecycle principles, data integrity, and digital lab tools reshape the regulatory [...]
February 6, 2026
If you oversee or participate in a medical product Stability program, you have been, or will be involved in an audit or inspection. While inspections [...]
January 11, 2026
So, your Stability Program is underway and/or flourishing. You’re focused on protocols, samples, chambers, disaster planning, testing, data analysis and reports—Each one gobbling up [...]
Share your questions and experiences
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!





