If you oversee or participate in a medical product Stability program, you have been, or will be involved in an audit or inspection. While inspections have the potential to bring your company’s operations and sales to a standstill, audits can cost you business partners and clients. The good news is that both can also lead you to higher standards of Good Manufacturing Practices and quality. It’s this latter scenario that we want to exploit in order to avoid those former unpleasant outcomes.
While preparing for the challenges of inspections, we’ll use audits as our tool to get there.
Worthy Goals
Make the Most of Stability Audits
Establish a realistic assessment of where you are in the preparation process.
Next, establish where the borders of your stability program are. You don’t have infinite resources and responsibilities, but as we’ll see, your program is connected to many stakeholders. Document your targeted Stability audit scope. It may include:
With your audit landscape mapped, what observations (internal, external, regulatory sources) / deviations and investigations have been made in this territory over the past 5 years and are any of those not completely corrected or prevented from occurring again? These documented liabilities are the first place auditors and inspectors will look upon their next visit, and repeat citations can quickly turn last year’s 483 into this year’s Warning Letter, or worse. Keep these at the very top of your list, even if they represent lesser risks.
In addition to in-house liabilities, it’s wise to look at what’s trending across the industry. FDA and other regulatory authority websites provide a wealth of information. Warning Letters and 483’s allow us to see common weaknesses in the Stability programs of other companies as well as the areas of focus and expectations of regulators. See if you can spot any liabilities you have in common with the unfortunate recipients of the selected examples below (paraphrased for brevity).
Returning to in-house considerations, what quality metrics could be “stability indicating” for your program?
With raised consciousness, it’s time to get analytical and mathematical.
Dare to engage in Risk Assessment
From your team and stability stakeholders, draw together your best unbiased thinkers to brainstorm for risk areas. Walk through your entire Stability process (A detailed process map is of great value in this exercise) to help jog people’s thoughts on gaps that they may have seen occasionally but hadn’t brought up till now. Review known risks to update or expand on them.
Ask the following questions about the equipment, systems, practices and people in your Stability realm:
- 1What could happen, go wrong with the equipment, systems, practices and people involved with the Stability Function? Establish each scenario of concern for risk assessment to apply the next 3 factors below.
- 2How likely is it to happen? (ex. 10 times in 365 days, 2% of run times, etc.)
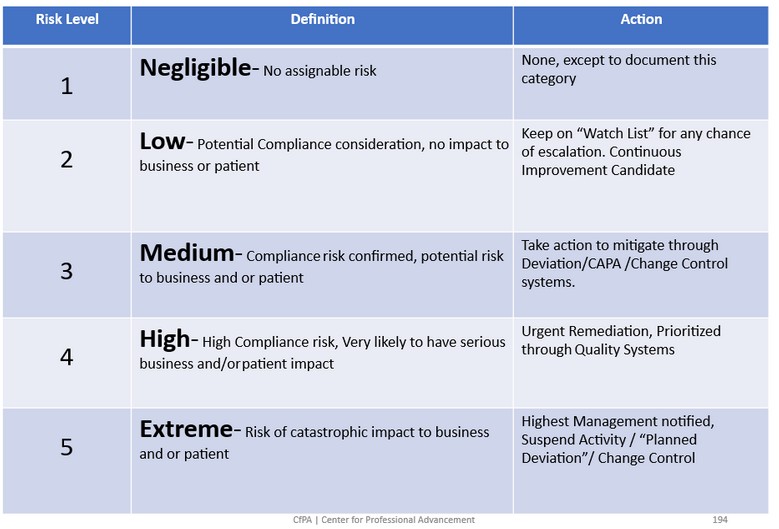
- 3What’s the Severity of impact on a scale of …? Establish a scale that best suits your need for standardized degree of severity, as in the example Five Tiered Risk Table below.
- 4What’s the likelihood of detection of the problem before significant impact?
Once quantified, plug these 3 values into the equation:
(Probability x Severity) / Likelihood of Detection = Degree of Risk
This process results in a list of potential liabilities that can be ranked by degree of risk, for remediation. A word of caution; prioritize with other significant factors in mind. Maybe a lower risk item has already drawn a 483 citation and needs to rise to the top of the list before it becomes a repeat observation. Maybe remediation of a higher risk item is dependent upon a system already under development and has to wait until its place in the process comes along. Be thoughtful in your remediation priorities, then proceed to eliminate or reduce the impact of the risks you’ve prioritized.
A Five Tier Risk Table
Perform a Walk Around Your Stability Area
Take some knowledgeable eyes with you.
Know the ins & outs of the chamber room function, such as security measures in place, security access criteria, reviews of authorization and entry, responsibilities for cleaning & repair and acceptable range for external room temperature and relative humidity.
Be Observant: What does an auditor see?
Simple aesthetics have a tremendous impact. A spick and span area tends to impress the auditors and helps to reduce the number of questions that could come up. Things start to get difficult when there are reasons for deeper discussion. Beyond cleanliness, can you explain:
What’s in those insect traps? How often are they checked, insects identified, and what is the housekeeping response?
Are there spaces under any exterior doors that you can detect light from outside? Are there ill-advised documents such as hand-written notes and discarded SOPs in the trash bins?
Is there unexplained apparatus on the walls (un-labelled pipes), counters or shelves?
Prepare for Justification of Decisions and Practices
You need to be prepared to justify the decisions and practices of your stabililty program. For example:
Justification for the number and placement of sensor probes. (controlling & monitoring)
Justification for the load patterns during Qualification
Adequacy of review and acceptance criteria for Qualification
Challenge All Aspects of Your Stability Program Justification
When an auditor asks “Why,” you should compare your answer against incremental justifications.
Along with periodic walk-throughs, have a special focus on SOPs. Maintain a complete list of all you use (vs. all you own). Identify key support SOPs referred to in your core SOPs.
Recent publications have stated that 40% of quality professionals are not confident that their SOPs are continuously audit-ready. Every regulatory change, quality event, or process improvement triggers a documentation cataclysm.
Assure that SOPs are accurate, updated and have documented regular reviews. A great tactic is to divide them up among staff and offer incentives for how many they discover to need updates or have gaps in them. This ensures multiple eyes on the SOPs and distributes the work.
Make sure QA/Inspection Management teams have latest versions.
Quiz your team on their Top 10 SOPs and their key provisions.
In addition to SOPs, pay attention to all the “Stability Quality Systems”, including:
Train, Practice, and Drill Stability Personnel
Test your team regularly until a real inspection seems less threatening for them. Select Stability audit hosts based on knowledge, poise and ability to think quickly and knowing when to defer to others. Have 2 or 3 trained hosts available to deal with audits on days when another host may be absent.
We need to be prepared to utilize technology to conduct many audit components remotely, when circumstances demand it. Plan for this contingency with your IT department and include this scenario in your training initiatives.
Keep the Audit Contained to Your Area
Many connections exist between the Stability Function and stability stakeholders. Work with the stakeholder be knowledgeable about their interface with your process. If you can answer some basic questions in this regard during an audit, you may avert a visit to the stakeholder’s domain by a curious or unsatisfied inspector. As a further precaution, stakeholder groups should have a knowledgeable member standing by to quickly show up in the Stability Area and keep the conversation geographically contained.
To optimize the Stakeholder audit partnership, compile a list of questions for your stakeholders.
Summary
Conducting effective Stability Audits includes having an accurate inventory of your Stability assets, systems and documentation, assessment of all activities and areas, securing a partnership with support functions, and monitoring trends through critical metrics. A well-trained and practiced team of audit hosts is necessary as well as a plan for virtual / remote audits. Continuous training, challenges and drill are keys to success.
References
Auditing Stability Programs: How to Ensure a GMP Compliant Program. Cobblestone Course ID 2801 Course Instructor John O’Neill. https://trainwithcobblestone.com/course/auditing-stability-programs-how-to-ensure-a-compliant-program/
Stability Program Software. SG Systems Global. https://sgsystemsglobal.com/guides/stability-program-software/
MasterControl Communication. MasterControl, 6350 South 3000 East, Salt Lake City, UT 84121
Share This Article with the Stability Community!
January 11, 2026
So, your Stability Program is underway and/or flourishing. You’re focused on protocols, samples, chambers, disaster planning, testing, data analysis and reports—Each one gobbling up [...]
January 11, 2026
In the tightly regulated world of pharmaceutical, medical device, and biologics manufacturing, contract storage facilities – warehouses or off-site stability storage facilities - play [...]
November 28, 2025
Hollister Incorporated, a global, employee-owned healthcare manufacturer, began a critical modernization initiative to overhaul its stability program. For years, Hollister had relied on traditional, [...]
Share your questions and experiences
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!





