In the tightly regulated world of pharmaceutical, medical device, and biologics manufacturing, contract storage facilities – warehouses or off-site stability storage facilities – play a vital role in ensuring product integrity over time. Whether housing active pharmaceutical ingredients (APIs), investigational products, or finished drug formulations, these facilities often fall under the oversight of the U.S. Food and Drug Administration (FDA). One important piece of FDA oversight is the Establishment Identifier, or FEI number.
This article outlines the circumstances under which a warehouse or stability storage facility may require an FDA Establishment Identifier (FEI) number and highlights its importance for regulatory compliance, submission of required documentation, and successful navigation of FDA inspections.
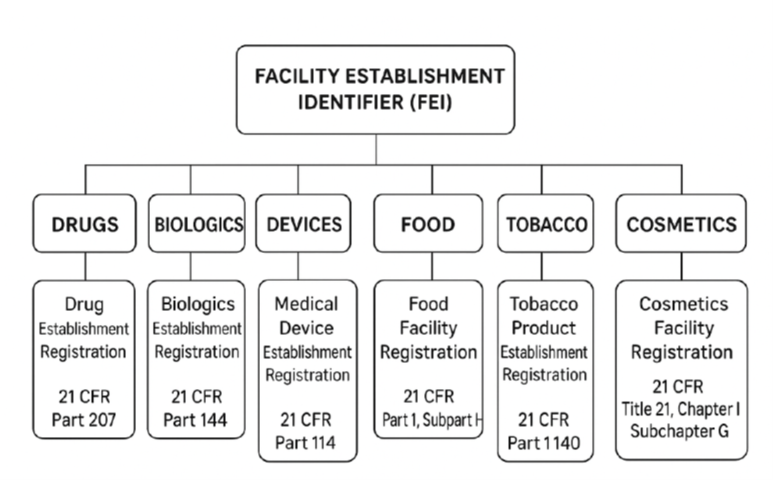
What Is a FEI Number?
An FEI number is a unique identifier assigned to facilities regulated by the FDA. The FEI number is assigned to a facility’s physical location address and remains the same across FDA centers (e.g., CDER, CBER, CDRH). The FEI number functions as the facility identifier used in inspection, regulatory filings, facility registration, and compliance actions. In contrast to a Drug Establishment Registration (DER) number, linked to annual registration obligations for drug manufacturers, the FEI number is permanent and linked to the physical facility location itself.
A single facility can have only one FEI number but be registered for multiple types of regulated products, such as: Drugs (CDER), Biologics (CBER), Devices (CDRH), Foods and Cosmetics (CFSAN), Tobacco (CTP), and Veterinary (CVM).
When Does a Contract Storage Facility Need a FEI Number?
While not every contract storage facility falls under FDA oversight, those that store materials related to FDA regulated products generally do require an FEI number. Your facility must be registered and obtain an FEI number if it meets any of the following criteria:
Your facility may not need to be registered if:
If the contract storage facility is part of the drug development, approval, or post-marketing surveillance ecosystem, obtaining an FEI number is not only expected—it’s often essential. If your facility is purely passive (non-GMP, non-submission-related), registration may not be required – but a risk-based assessment and legal review are recommended.
Interpretation
The FDA’s “storage-only facility” exemption is not absolute — it depends on whether the storage operation is passive or involves activities that would cross into “processing, testing, control, labeling, handling” or other regulated functions.
Even for drug products, if a storage facility were to perform quality control, sampling, testing, stability test pulls, or other operations that might be considered part of the “control” or “manufacture” chain, FDA might deem that facility as within the scope of registration. The 2016 rule’s language suggests that if you go beyond mere storage, the exemption may fall away.
It can also depend on the product type (drug, biologic, food, medical device) and whether other statutes (e.g. biologics, device establishment registration) have different rules.
Guidance or internal FDA policy interpretations may also affect how strictly the storage-only exemption is applied. Sometimes, in practice, FDA may expect registration for storage operations closely tied to regulated products or stability programs, especially if stability is critical to product quality oversight.
In Plain Language
Manufacture under FDA law doesn’t just mean making a drug. It also includes handling, testing, sampling, controlling, or otherwise affecting a drug’s quality, safety, or labeling at any stage of its lifecycle. Even if a company performs these activities on behalf of another manufacturer, it is still considered a manufacturer and must register with the FDA.
Handling: Refers to the physical receipt, movement, storage, protection, or manipulation of components, in-process materials, or finished products in a way that can affect their quality, identity, strength, or purity. Example: A facility that opens sealed stability samples to pull test units is handling the product under FDA’s definition – therefore, that activity becomes part of the manufacturing operation and may require establishment registration.
Sampling: The systematic removal of a representative portion of material or product from a defined population, batch, or container for the purpose of testing, inspection, or verification of quality attributes. Under 21 CFR 207.1, “manufacture” includes sampling because:
Example: A warehouse that stores GMP stability samples and pulls samples monthly for laboratory analysis is performing a manufacturing support function and must be registered as a drug establishment under 21 CFR Part 207.
Controlling (Control or Control Procedures): Refers to systematic actions that ensure product quality, identity, strength, or purity, and performance. When the FDA refers to “controlling” a product or process, it means performing checks, tests, or verifications that ensure it meets approved specifications and remains in a validated, compliant state. Because these activities determine product quality and compliance, the FDA considers control procedures to be manufacturing operations under 21 CFR Part 207, requiring registration and cGMP compliance when performed as part of drug production, testing, or stability programs.
In the FDA document, Identification of Manufacturing Establishments in Applications Submitted to CBER and CDER – Questions and Answers – Guidance to Industry (October 2019):
Question 1: What facility information should I include in the establishment field on Form FDA 356h?
Response (from the FDA):
For original NDA, ANDA, and BLA applications, amendments, efficacy supplements, CMC supplements, and resubmissions to these submission types, the applicant should include complete information on the locations of all manufacturing, packaging, and control sites for both substance and drug product on Form FDA 356 h. This should include:
All facilities used for storing or warehousing drug substance, in-process material, and commercial drug product under quarantine prior to a disposition decision, including any facilities that solely store the stability samples.
In the Federal Register Vol.81, No. 169, August 21, 2016, Requirements for Foreign and Domestic Establishment Registration and Listing for Human Drugs, Including Drugs That Are Regulated Under a Biologics License Application, and Animal Drugs:
C. Registration (Part 207, Subpart B) 1. Who Must Register? (§ 207.17) (Comment 26): One comment asked FDA to clarify whether a storage facility that does not repack or relabel drugs is required to register under part 207. (Response): A facility at which drugs are stored, such as a warehouse, does not need to be registered provided drugs are not manufactured, repacked, relabeled, or salvaged (as those terms are defined in § 207.1) at the facility. Note that the definition of “manufacture” including sampling, testing, or control procedures applied to the final product or to any part of the process. Thus, for example, if a warehouse includes a temperature-controlled storage area where drug samples are stored for stability testing to satisfy current good manufacturing practice requirements, that activity would qualify as a manufacturing operation and require registration of the warehouse as a drug establishment. Other State or Federal Requirements may apply to such facilities.
What if the Contract Storage Facility is not Registered with the Correct Business Center?
As stated previously, FEI numbers are facility specific not product specific. So, the FEI number can be used across biologics, drugs, and devices, provided the facility is properly registered for the applicable activities.
The facility must be registered correctly for the type of product and activity it performs:
Some potential consequences of using a facility that is not properly registered with the appropriate FDA Business Center, yet listed in an NDA, ANDA, or BLA, include the following:
Regulatory Submission Rejection
If the facility is listed in an NDA, ANDA, or BLA, the FDA may issue:
This occurs because the registration does not fulfill the requirements (e.g., drug establishment regulation does not fulfill the biological registration requirements under 21 CFR Part 600).
Inspection and Compliance Delays
FDA inspectors rely on accurate establishment registrations to schedule pre-approval or surveillance inspections. Without proper registration, inspections may be delayed, rescheduled, or escalated, impacting approval timelines.
Audit Findings and FDA 483 Observations
During client or FDA audits, failure to register the facility properly may result in:
Regulatory Non-Compliance
The facility is considered non-compliant with 21 CFR §207.17 and §207.41, which require that any facility engaged in the manufacture, preparation, propagation, compounding, or processing of a drug – including stability storage under GMP – must be registered.
Even though “biological establishment registration” (21 CFR Part 600) may exist, it does not satisfy the requirements for a drug product regulated under CDER.
Delayed Product Approvals
Any product referencing the improperly registered facility in its submission may have its review timeline extended, especially if the FDA must clarify or correct registration status.
Legal and Commercial Risk
Clients that depend on your facility’s registration may choose to terminate contracts or relocate stability programs if your registration status leads to regulatory delays or poses a compliance risk.
From a legal perspective, operating without the appropriate FDA registration may constitute a violation of the Federal Food, Drug, and Cosmetic Act (FD&C Act).
Simply put, lacking an FEI number when one is required or not registering with the correct center, can limit your facility’s role in the drug supply chain and negatively impact client relationships.
Prior to referencing any facility in regulatory submissions (NDA, ANDA, or BLA), confirm that registration with the appropriate FDA Center is complete to mitigate potential compliance risks.
Practical Considerations and Caveats
Registering Your Facility and Obtaining an FEI Number
Below are the typical steps in obtaining an FEI number.
Notes:
-
FEI is used in inspections, drug listing, and FDA correspondence, not for commercial activities.
-
Annual renewal is required between Oct 1 – Dec 31 every year.
Conclusion
For any contract storage facility involved in FDA-regulated product storage, whether as a stand-alone service or part of a broader manufacturing operation—an FEI number is a crucial component of compliance. It enables end-to-end traceability across the supply chain, addresses regulatory submission compliance, and strengthens facility reputation and adherence with industry standards across the pharmaceutical landscape.
If your facility provides stability storage services and has not registered for an FEI number, now is the time to assess your regulatory compliance and engage with the FDA. The cost of non-compliance is high—but the solution is well within reach.
Authors
Kenneth L. Edwards and Monica B. Edwards, of Concentric Biopharmaceutical Services, Inc., bring extensive experience in regulatory compliance, validation, and quality systems within FDA-regulated industries. Their collaborative work helps organizations interpret and apply FDA and ICH requirements across contract facilities, stability programs, and computerized systems.
At Concentric Biopharmaceutical Services, they lead client initiatives in GxP compliance, FDA registration accuracy, and data integrity assurance, supporting pharmaceutical, biologics, and medical device operations across the development and commercial lifecycle.
References
FDA Drug Establishment Registration and Listing Rule
Title: Requirements for Foreign and Domestic Establishment Registration and Listing for Human Drugs, Including Drugs That Are Regulated Under a Biologics License Application, and Animal Drugs
Federal Register: Vol. 81, No. 169 — August 31, 2016 — Rules and Regulations (Page 60184)
Identification of Manufacturing Establishments in Applications Submitted to CBER and CDER – Questions and Answers (Guidance for Industry, October 2019)
Relevant CFR (Code of Federal Regulations) Sections
21 CFR 207.41 – Who must register
Specifies that establishments engaged in the manufacture, preparation, propagation, compounding, or processing of drugs must register with the FDA. It includes some types of warehousing or storage when associated with drug manufacturing functions.
21 CFR 207.49 – What information must be submitted
Details the required information for drug establishment registration, including FEI number-related data.
FDA FEI Number Database
FDA FEI Search Portal (used to confirm and validate FEI numbers assigned to establishments):
https://www.accessdata.fda.gov/scripts/cder/fei/index.cfm
FDA Drug Registration and Listing Instructions
Provided by the FDA’s CDER (Center for Drug Evaluation and Research):
FDA Registration and Listing System (DRLS) and eDRLS Instructions
https://www.fda.gov/drugs/drug-registration-and-listing-system-drls
FDA eDRLS System (Electronic Drug Registration and Listing System)
Portal used for submitting establishment registration and listing information:
https://www.access.fda.gov
Share This Article with the Stability Community!
January 11, 2026
Your Stability Program is focused on protocols, samples, chambers, disaster planning, testing, data analysis and reports. Any one of these areas can be a [...]
November 28, 2025
Hollister Incorporated, a global, employee-owned healthcare manufacturer, began a critical modernization initiative to overhaul its stability program. For years, Hollister had relied on traditional, [...]
November 1, 2025
It happens. Your stability chamber malfunctions or quits altogether and your product’s target exposure range has a significant deviation: too hot, too cold, too [...]
Share your questions and experiences
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!





