Disasters occur whether we plan for them or not, but planning ahead to mitigate their impact and recover from their damage can make a big difference in corporate success. While we can’t control the nature or the timing of such events, we usually have ample opportunity for risk assessment and developing potential courses of action. The real tragedy is that we often delay or de-prioritize taking the necessary measures.
While not having specific regulations requiring disaster plans, the FDA strongly encourages all medical product stakeholders to develop and maintain such plans for emergencies, aiming to minimize disruptions to patient care and ensure the continued availability of essential medical products. Disaster plans should be adaptable to various situations and allow for quick adjustments based on evolving circumstances.
Key Elements of an Effective Disaster Recovery Plan
Key elements of any effective recovery plan should include the following:
What Types of Disasters?
Disasters can vary depending on the location of your business, the relationships that you share (at a national, international, and business-level), supply-chains in place, and many more.
There are, however, some classic disasters that you should have on your list. These include:
How Do I Begin Planning?
As in any planning endeavor, getting the right people into the room and conducting a thorough risk assessment exercise is a natural beginning. It’s a great advantage to mine the experience of as many stakeholders as possible. There are more than 30 Stability Stakeholders/functions to consult regarding disaster planning. The war stories of industry colleagues beyond our walls are equally valuable.
This is the standard equation to determine the degree of risk:

The greatest challenge is arriving at an acceptable scale to use for each of the terms, but once established, we have a great tool at our disposal. In Disaster Planning, there’s an advantage in the Probability of Occurrence category due to the availability of data regarding natural events in your area as well as trending of equipment problems. In many instances, Likelihood of Detection is high with respect to weather warnings, fire detection, pandemics, etc., but low for earthquakes and computing/hacking events. Degree of Severity would be maxed out in the event of chamber and sample loss, but much lower in those cases where samples are preserved for alternate conditioning.
As an example, here is a quick take on each of the various risk categories, with the assumption that a scale has been developed by the User to assign high, medium, and low ranges to each of the factors. Adjust for your own circumstances and add pertinent risks not listed below.
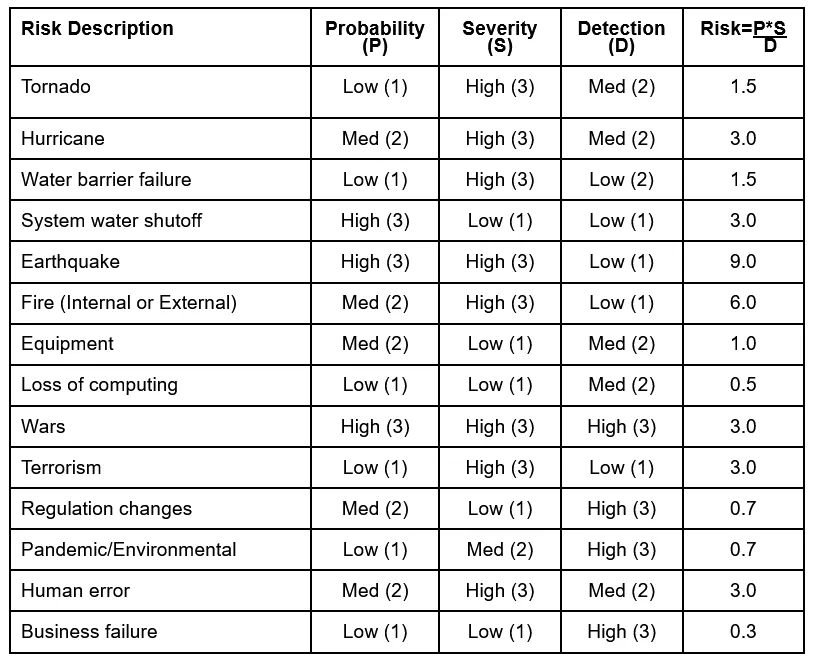
With risk levels established, priorities for remediation can be set. While logical to address them in highest to lowest order, other factors such as cost, regional susceptibility, and recent occurrences may change the order in which you wish to tackle them.
Detection and Prevention Best Practices
It’s said that an ounce of prevention is worth a pound of cure. Where possible, advanced preventive measures as well as improved ability to detect a threat are effective strategies.
While probability of natural events generally can’t be influenced, any internal risks such as water, electrical, human error and computing can be mitigated through improvement initiatives.
In earthquake zones, barriers on shelf edges, and tie-down straps go a long way in sample preservation.
Chamber area rooms should have floor drains and moisture monitors to detect and channel water away from chambers.
Engineering awnings, catch shelves, and diversion channels to protect in-chamber samples residing under water piping is also a great preventive measure.
Regular maintenance is essential to minimize equipment failures.
Chamber back-up measures are prudent through having a qualified, empty, operating chamber available nearby or alternatively have a contract arrangement with an off-site GMP storage facility a prudent distance from the risk zone.
Detailed procedures and frequent training in both disaster preparedness and response lessen the impact of disasters and hasten a return to normal operations. Can you pack up your stability samples and protect them for transport with an hour’s notice? Periodic drills with simulated samples (for due caution) and involving all potential participants from packers to receivers could be a wise investment. Frequently backing up protocols and data in the cloud is also a best practice to preserve information when fire, wind, flood or hackers intervene. To diminish the possibility of human error, leave no loop open, no receipt unsigned, no check point unchecked.
Why isn’t disaster preparedness more prevalent or more sustainable?
The most complete disaster plans are expensive to implement and maintain. In the absence of disasters over the course of time, corporate cost cutting and conservation measures will bring the scrutiny and fiscal knives of the budget crew who will point out that the Disaster Planning measures have not been utilized in quite some time. Taking a Disaster Planning poll among industry colleagues often brings the response, “We used to have a plan, but it was discontinued.”
Preserving Your Plan
Aim for the most cost-effective measures when drafting a plan. Having an annual contract with a secondary storage facility (and making at least minimal usage of it) may be cheaper than buying a back-up chamber or two and keeps your line item out of the capital equipment category.
Have a vigorous defense ready for your plan, citing risk and cost factors associated with the loss of chambers and stability study samples upon which success of a new product registration lies.
Summary
Assessing risk, mitigating liabilities, planning responses and practicing for every scenario is an effective approach to disaster planning. Continuous diligence and involving all stakeholders ensures the most effective response when the (formerly) unthinkable comes to pass. Do you have a comprehensive and well-practiced plan?
Share This Article with the Stability Community!
February 6, 2026
If you oversee or participate in a medical product Stability program, you have been, or will be involved in an audit or inspection. While inspections [...]
January 11, 2026
So, your Stability Program is underway and/or flourishing. You’re focused on protocols, samples, chambers, disaster planning, testing, data analysis and reports—Each one gobbling up [...]
January 11, 2026
In the tightly regulated world of pharmaceutical, medical device, and biologics manufacturing, contract storage facilities – warehouses or off-site stability storage facilities - play [...]
Share your questions and experiences
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!





