So, you’ve gone through the process of formulating user requirements, chamber selection and purchase. With the equipment in place; how do we go from this point to GMP operation? Are we required to perform validation or qualification? What about calibration and requalification? The answer is yes to all of these, but there is much involved as to how these are implemented.
What’s the Difference Between Qualification and Validation?
The terms validation and qualification are often used interchangeably but have distinct meanings and serve different purposes. Understanding the differences between qualification and validation is crucial for anyone in the pharmaceutical industry.
While qualification focuses on verifying the fitness of equipment and facilities, validation ensures that processes consistently deliver expected outcomes. There is a general saying:
Qualify a system and/or equipment and validate a process. A system and/or equipment must be qualified to operate in a validated process.
The following illustration may help visualize the difference.
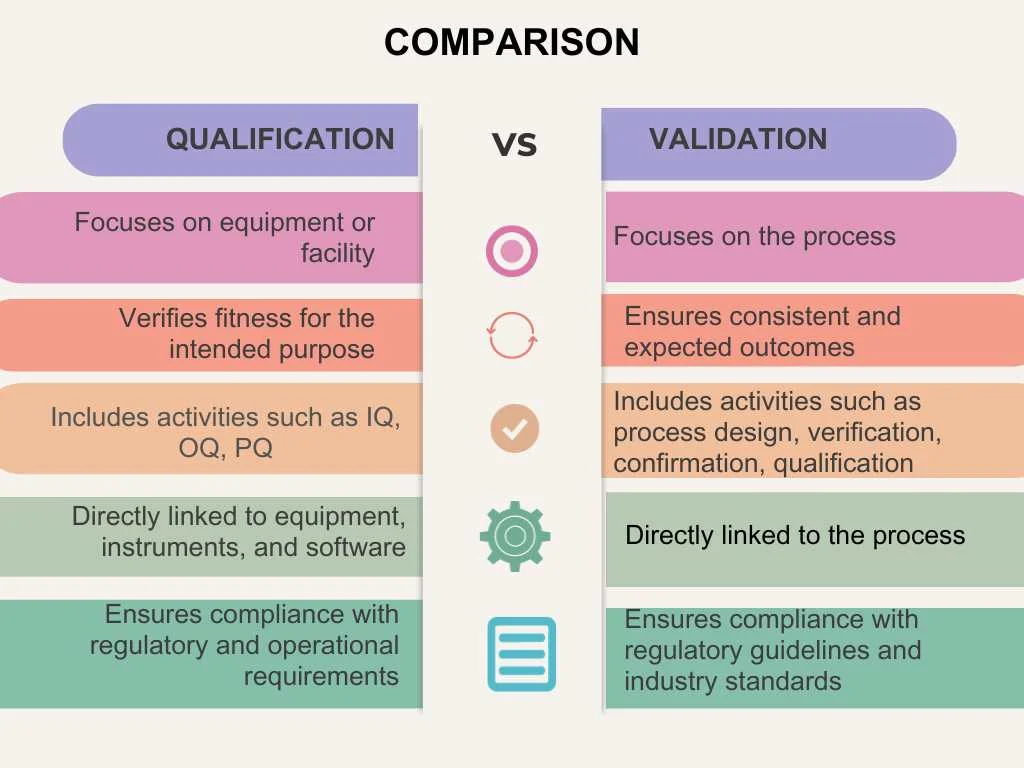
Validation
A stability chamber validation protocol ensures that a controlled environment maintains specified conditions consistently over time, supporting reliable product stability studies. The validation process typically includes:
- Creation of a Validation Plan: Define objectives, methods, and anticipated barriers.
- Selection of Measuring Instruments: Choose appropriate tools like thermocouples, RH sensors, and data loggers.
- Preparation and Use of Instruments: Ensure instruments are calibrated and placed according to the protocol.
- Data Recording and Storage: Capture and store data from the sensors.
- Data Evaluation: Analyze data to determine if the chamber meets specifications.
- Final Documentation and Release: Document findings and release the chamber for use.
Qualification
Qualification is a comprehensive process applied to various elements within a manufacturing facility, encompassing:
- Equipment: Ensures the correct functioning and performance of manufacturing or testing equipment.
- Ancillary Systems: Validates the reliability of support systems integral to the manufacturing process.
- Instruments: Verifies the accuracy and precision of measurement instruments used in production.
- Utilities: Qualifies utility systems such as water, air, and power to meet specified requirements.
Tenny Environmental puts stability chamber qualification requirements this way:
For an environmental chamber to receive a certification to conduct stability tests, it must undergo a three-part qualification process and include calibration records of all instrumentation used and evidence of their calibrated state. Together, the following three qualification requirements and tests support and document proper operation.
1. Installation Qualification (IQ)
Overview: The first qualification verifies whether or not the stability chamber meets its design specifications. All parts are accounted for and checked that they are correctly installed. Proper documentation must also be present, including the user manual and any certifications and standard operating procedures (SOPs).
Requirements: All components must be properly installed and functional for a stability chamber to pass installation qualification. If the chamber has a control panel with a human-machine interface (HMI) or other pilot devices such as pushbuttons and selector switches, they must work as designed. All calibration and safety equipment also need to pass inspection.
2. Operational Qualification (OQ)
Overview: The operation qualification tests the individual functions of the stability chamber and verifies that the systems and subsystems perform as intended under normal operating ranges—including testing all doors, switches, controls, and alarms. Involves temperature and/or humidity mapping of the empty chamber to confirm it operates within specified limits throughout.
Requirements: The operation qualification test verifies that temperature and humidity have equalized inside the chamber. To ensure accurate temperature and humidity readings, the operator waits a few minutes until they stabilize, then runs the test for the specified time. Includes calibration of measuring equipment and defining thermocouple/data logger locations within the chamber.
A door opening study is performed before completing the operation qualification. This test involves opening the door for a short period while recording temperature and humidity in short intervals. The door open trial has been repeated a minimum of three times to calculate the chamber’s average recovery time.
3. Performance Qualification (PQ)
Overview: The performance qualification verifies that the stability chamber meets performance specifications under a full load. Usually, the test is performed at the chamber’s operating setpoint with a simulated product loaded to replicate a typical environment. Temperature and relative humidity uniformities are measured using thermocouple and relative humidity (RH) sensors. Involves monitoring for alarms and other indicators of malfunction.
Requirements: The stability chamber must perform under full load for at least 24 hours to pass performance qualification. After the initial study is complete, the trial gets repeated with a door-opened study to calculate its average recovery time.
Chamber Mapping
Chamber mapping is used to verify that the chamber temperature and/or humidity distribution consistently remains within the specified tolerances by taking readings in a predetermined pattern throughout the chamber over a specified period of time. Any persistent high or low readings at a particular location identify problem areas that can then be addressed. Mapping is a crucial part of qualification and initially occurs during the Operational Qualification (OQ) phase with an empty chamber. Mapping is repeated during the Performance Qualification (PQ) phase with a “loaded” chamber containing materials that represent the anticipated operating load of the chamber and is also repeated during subsequent chamber Requalifications (RQ) and chamber investigations.
Number and location of sensor probes may vary by size of the chamber being monitored, but in general, probe locations should generally include all corners, the geometric center, and the center of each side, with a distance of at least 10 cm from the surfaces. Additional probes should be placed at the control sensor location, and in high-risk areas like near doors or HVAC vents. Keep sensors away from walls to measure the true usable storage space, not the chamber’s dead zones. It’s important to calibrate the sensors before and after the mapping study, especially if using thermocouple-based systems.
The following are good resources for chamber qualification and mapping.
- ISPE Good Practice Guide: Controlled Temperature Chambers, 2nd Edition The Guide has been revised to align with current industry practice, particularly with respect to the ISPE Baseline® Guide: Commissioning and Qualification (Second Edition) (involves purchase)
- Vaisala Application Note- 8 Steps to Validating/Mapping a Chamber
Calibration
Proper calibration is crucial for accurate and reliable stability testing results, ensuring the validity of shelf-life determinations and other stability studies. The calibration of a stability chamber involves verifying its performance against a set of standards and making adjustments as necessary to ensure that it meets the desired specifications.
Regarding calibration for temperature and/or humidity:
- Temperature calibration: A reference thermometer is placed inside the chamber and compared to the chamber’s temperature readings. Adjustments are made until the readings match, ensuring accurate temperature control.
- Humidity calibration: A reference hygrometer is used to verify humidity readings. The chamber’s humidity control system is adjusted until the readings match, ensuring accurate humidity control.
Calibrate each chamber to NIST (National Institute of Standards and Technology) compliance , in order to meet and exceed manufacturer’s specifications. This is done by placing a calibration probe in the center of the chamber and comparing the temperature reading of the probe to that of the chamber’s display. Adjustments can then be made to the display and using this method, the chamber can be calibrated to get as close to the stated tolerance as possible.
Provide a 3-point “as-found” calibration verification. The “as-found” readings are documented and verified to be within the manufacturer’s tolerance, if found within the tolerances the device is then returned to service. Any instrument outside of tolerance must be noted, and appropriate actions taken to bring it into satisfactory calibration
If an instrument’s “as-found” readings are outside of the recommended tolerance level, the instrument can be calibrated using the manufacturer’s calibration procedures. After completing the calibration, the instrument’s “as-left” readings are documented using the same three points from the calibration verification to ensure the instrument is reading within the manufacturer’s tolerance. Final documentation upon completion includes the chamber’s performance within the manufacturer’s tolerance, including the readings of the unit during operation.
Chamber calibration intervals can vary according to need and justification, but should never be longer than annually and are more typically performed at 6 months with fallbacks to 1 and 3 months depending on changes to chamber parts, settings, load patterns and any other disrupting influence, including failure to meet a current calibration.
Requalification
Stability chamber requalification is the periodic assessment of a stability chamber to ensure it continues to operate within specified limits and maintains its accuracy, uniformity, and reliability at specified intervals after initial qualification or following changes that could impact its performance.
Over time, stability chambers can experience drift or changes in performance due to various factors like significant changes to the chamber such as component wear, repair, modifications or component replacement, load distribution, relocation and changes of setpoints.
Many regulatory agencies require periodic revalidation or requalification of equipment used in pharmaceutical stability testing. Requalification should be performed on a regular basis and/or after any significant changes to the chamber such as those mentioned above.
Periodic re-mapping ensures temperature/humidity uniformity and identifies any emerging hot/cold/wet/dry spots. After each mapping/requalification, the results should be compared with previous years to track the chamber’s overall performance.
When should requalification be performed?
It depends: Some follow various recommendations to requalify/remap annually. The WHO Supplement 8 (Temperature mapping of storage areas, Technical supplement to WHO Technical Report Series, No. 961, 2011) to Annex 9- Model guidance for the storage and transport of time and temperature-sensitive pharmaceutical products (May 2015) recommends requalifying every 2-3 years if no other change factors intervene. PSDG polling shows that best industry practices typically reflect the range of 1, 2, or 3 years when the change aspect doesn’t kick in. Others report “as needed”, or in other words, only when a change occurs. To our knowledge, there have been no regulatory repercussions to any of these choices with appropriate justification unless a neglect of any of the noted chamber change factors has been evidenced.
Summary
Once Stability Chambers are purchased and arrive on site, a detailed Validation plan is required to achieve GMP Compliance as well as successful operation. A group including the User, HVAC/Maintenance, Validation Team, Metricians, QA, Maintenance, Information Technology, Training and Security should draft an acceptable comprehensive plan and execute it according to published standards.
Share This Article with the Stability Community!
February 6, 2026
If you oversee or participate in a medical product Stability program, you have been, or will be involved in an audit or inspection. While inspections [...]
January 11, 2026
So, your Stability Program is underway and/or flourishing. You’re focused on protocols, samples, chambers, disaster planning, testing, data analysis and reports—Each one gobbling up [...]
January 11, 2026
In the tightly regulated world of pharmaceutical, medical device, and biologics manufacturing, contract storage facilities – warehouses or off-site stability storage facilities - play [...]
Share your questions and experiences
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!





