Every stability study has one (unless maybe you are a stability study featured in a Warning Letter). A protocol captures the design of a stability study, but should it 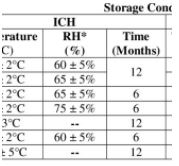
When searching for a document that best describes the design of your stability study, what better place to go than a well-constructed protocol; built on science, compliance, statistics, timing and nature of registration and justification?
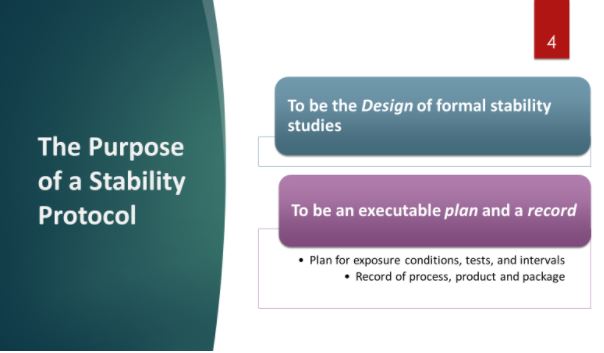
On the other hand, if we fail to build a solid protocol and not manage change control, we risk:
- Disconnects to the “story” of our product
- Ambiguity of process and container-closure details
- Limited scope of design, omitting special international requirements
- Missing input from supporting key disciplines
- Missing revisions and changes in aspects such as Test Methods and Specifications
- Late additions of Contract Research Organizations may not be noted and tracked.
- Receiving a regulatory citation or Warning Letter that announces our protocol failures to the world
As stabilitarians, what elements of quality can we add to our own corporate stability protocols? Chime in (with a free account) at our discussion board!
John O’Neill
Editor, StabilityHub
Share This Article with the Stability Community!
June 2, 2025
So, you’ve gone through the process of formulating user requirements, chamber selection and purchase. With the equipment in place; how do we go from this [...]
April 26, 2025
It’s Stability Information Month and time to go a little further than regulations and processes and talk about people, namely the ones who comprise the [...]
March 30, 2025
In the pharmaceutical, food, and chemical industries, product stability is paramount. Ensuring that a product maintains its quality, potency, and safety over its intended [...]
Share your questions and experiences
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!
A stabilitarian encounters new situations every day. StabilityHub’s discussion forums give Stabilitarians an opportunity to ask questions and offer solutions to specific scenarios. Join in the conversations with other Stabilitiarians and share your knowledge!





